GPS session help guide
Getting ready for your GPS session
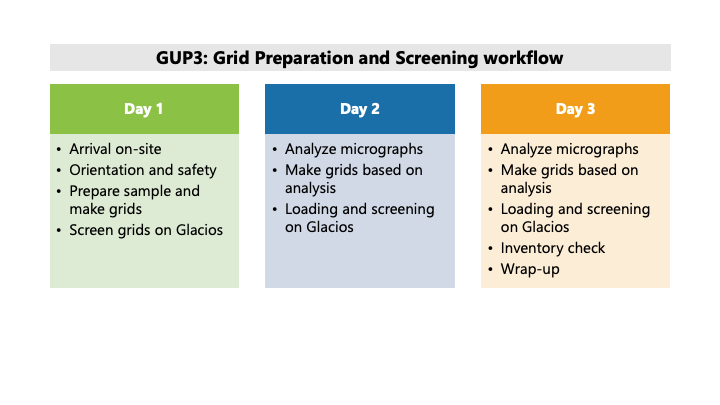
You have been assigned a Grid Preparation and Screening (GPS) session at NCCAT. What are the next steps?
Make sure you have completed the pre-session forms:
[User registration][Shipping manifest][Shipping agreement]Review our shipping guide [here].
NCCAT provides common cryo-EM consumables. Please ensure you ship or bring non-cryo-EM or bespoke cryo-EM consumables you might need.
Verify the spokesperson of the proposal can login with your account at nccatweb.nysbc.org.
Register an account with Globus.org so you may download your data. See our quick guide or Globus’ FAQ.
If you’re coming on site, prepare a small file (e.g. 3-5 Powerpoint slides) as a springboard for discussing with NCCAT staff at the start of your on-site visit:
– the sample’s molecular weight and concentration
– basic biochemistry (e.g. final purification gel and chromatography profile)
– exemplar images from any previous cryo-EM attempts (raw micrographs, 2D classes, 3D volumes)
– list some existing relevant literature of cryo-EM on the same/similar sample
Check out our online resources here.
Sample or grid requirements:
1. If sending samples:
The amount of sample required for 3-4 days of screening and optimization largely depends on the sample itself, the types of challenges it faces, the optimization steps required. As a ballpark starting point, we recommend:
Sample concentration: > 2 mg/ml
Sample volume: > 1 ml
Buffer volume: 100 ml
This is a general recommendation aimed to allow for trying as many screening conditions as possible in 3-4 days, and not a rule. We will work with you and your sample to determine a suitable amount of samples to prepare for the GPS session.
NCCAT provides common cryo-EM consumables. Please ensure you ship or bring non-cryo-EM or bespoke cryo-EM consumables you might need.
2. If sending grids:
You may send up to 24 grids in 6 boxes. Your grids will be automatically screened on our NCCAT Glacios with Smart Leginon, with a maximum of one cassette loading in the morning and one in the evening.
When you are on-site
1. If you sent samples:
A customized three- or four-day plan for screening and optimizing your samples will be developed together with you during your pre-session meeting.
A staff member will work closely with you when you’re on site to screen and optimize your sample for cryo-EM. In general, we will be aiming for the following workflow:
1. Prepare sample with generic grid-preparation conditions.
2. Image sample on a TEM and analyze images to understand potential issues.
3. Optimize sample preparation conditions.
4. Repeat steps 2-3.
From experience, grid-making conditions for 1-2 samples can be optimized in this 3 to 4-day period, during which 15~30 grids are likely to be prepared under various conditions, depending on the needs of the sample.
You will have access to all the sample optimization tools available on-site, including carbon and gold grids, graphene grids, Vitrobot, the Chameleon, and Refeyn. We also have a buffer optimization screening kit, and a fully equipped wet lab. NCCAT provides common cryo-EM consumables; please ensure you ship or bring non-cryo-EM or bespoke cryo-EM consumables you might need.
In a three-day visit, we will aim to complete three cycles of the screening workflow with the goal of preparing Krios-ready grids. At the end of your visit, we will review the findings together and put together a plan for next steps. If more biochemical optimization of your sample or data processing at your home institution is required to improve or better understand the sample, one supplementary visit to NCCAT can be arranged once the optimization and processing steps are complete. There will be another pre-session meeting ahead of the supplementary visit to plan resources and strategize.
2. If you sent grids:
Your grids will be automatically screened on our NCCAT Glacios microscope with Smart Leginon. Your data can be viewed by using the Leginon and Appion webtools as detailed below. You can choose to review your data with us after the session.
Leginon and Appion webtools
nccatweb.nysbc.org
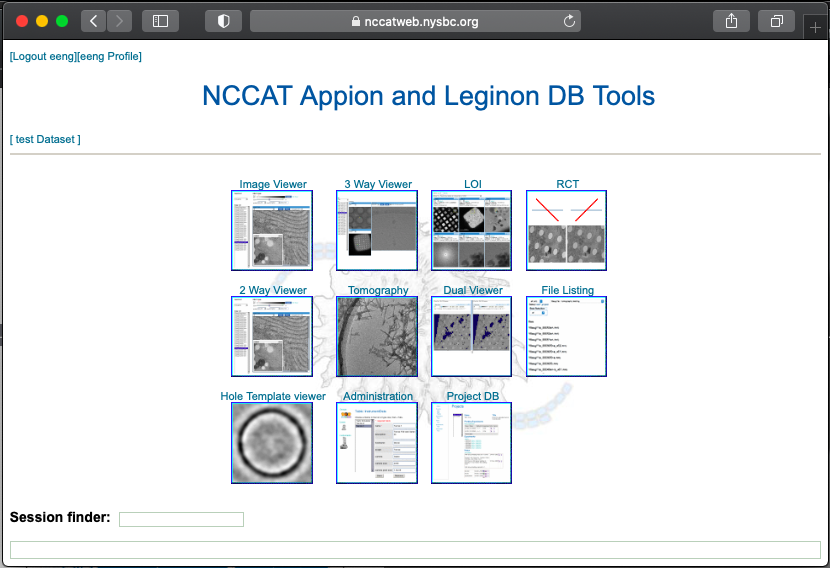
At NCCAT we use Leginon for data collection. For responsive feedback, Leginon has webtools so the user may monitor the data collection and be able to also analyze the quality of the grids made.
By default, we collect data under the spokesperson’s account. If additional accounts are required, these requests may be made to the NCCAT User Office during your pre-session meeting, or email correspondences, ahead of a scheduled data collection session.
If you have trouble with your accounts you may email the NCCAT User Office.
After your session / getting your data
Please download your data via Globus. It would be easier to download the raw data after a session is finished, but the Leginon aligned sum images (*enn-a-DW.mrc) are relatively small and that would be useful if you wanted to use them for initial processing feedback. We will store your data for 1 month, then it will be queued for deletion. If you have issues downloading your data please let us know.
Your data is only accessible through the spokesperson’s login located in a few places:
- the data on nccatweb.nysbc.org is located in /beegfs/leginon/[username]/ ,
- the direct detector data (movies) is located in /beegfs/frames/[username] ,
- the pre-processing is in the Appion folder and may be regenerated through processing and located in /beegfs/appion/[username] , and
- the references are located in the folder called references in the frames directory. You may see a couple because we updated the gain after we started the session. So the latest one is the one to use.
Data directories
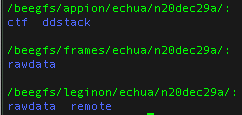
Note: At minimum users should always download all the data in the frames folder because this folder contains your raw data and all other files may be regenerated from your raw data.
References
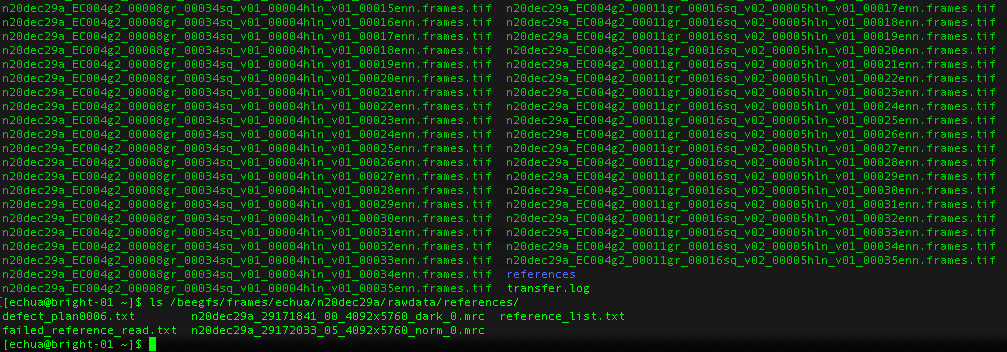
What about gain references?
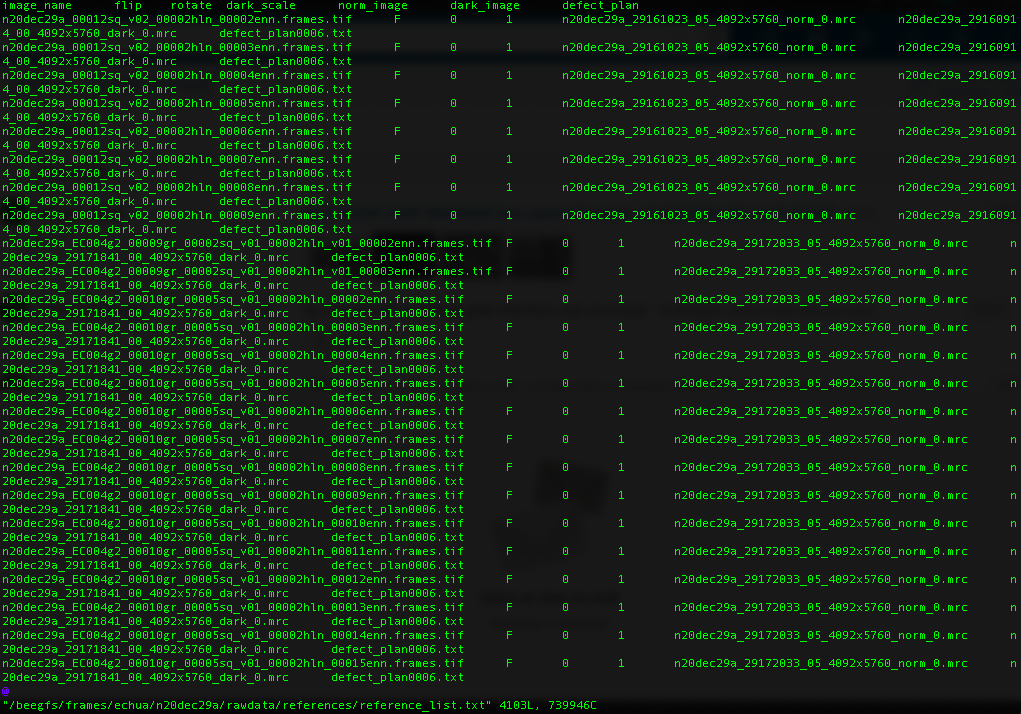
For Gatan K3 images gain correction requires a y-flip for most motion correction programs. The K3 is hardware dark corrected and only the gain reference is needed. The name of the gain reference would be in a format, such as “n20dec29a_29172033_05_4092x5760_norm_0.mrc”. If there are multiple references you should use the latest. If a new gain reference was taken in the middle of the session it would be annotated in the” reference_list.txt” file.
For Falcon III and Falcon IV data, there are no gain references required since the images are hardware gain and dark corrected.
For more information read our wiki [here].
Reference list for K3 data
We use Globus, a non-profit service for secure, reliable research data management, for data transfer.
Please note that you are responsible for your data and samples. In general, direct detector data will be queued for deletion in a month. Your Leginon images are not backed up and may be converted to JPEG versions within 1 year. We will make our best efforts to preserve the functionality of the image viewer, but do not archive data. If you are having trouble with Globus let us know. If it is the endpoint we maintain our IT may assist, but Globus is a service we subscribe to and general help may also be found on their website: https://www.globus.org/
Globus file manager
At the end of your session
At the end of your session please make sure to contact the NCCAT User Office to work out any sample/grid logistics and fill out the end of session forms.
